Lean Pharmacovigilance: Drug Safety Enhanced By Lean Culture
Pharmacovigilance is Preventative Pharmaceutics
|
Since its inception, pharmaceutical manufacturing has been highly reactive in nature resulting in implementing processes that in some cases increase the risk for compliance failure. Pharmacovigilance is the proactive detection, assessment, understanding and prevention of any drug-related problems where prescription medications are concerned.
The implementation of a Lean Pharmacovigilance culture can help your company dramatically reduce quality costs and improve compliance performance by streamlining its drug safety protocols—resulting in safer medications, sustained operational improvements and increased profit margins. |
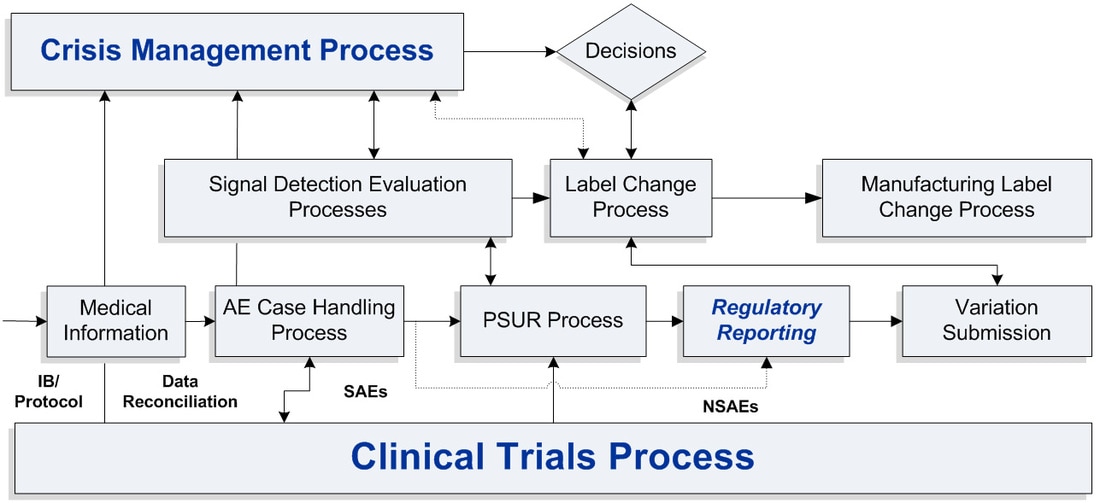
Drug Safety (Pharmacovigilance) is the practice of receiving, reviewing, assessing and reporting adverse events from drugs produced and administer during the different phases of clinical trails and post-marketed studies. The main objective of Pharmacovigilance is two-fold; (1) Legislative Compliance, Periodic Safety Updates, Registration, SERM, Product Safety Updates, CIOMS and Medwatch and (2) Protect the patient and the company. The Center for Drug Evaluation and Research (CDER) in the United States is responsible for evaluating the safety profiles of drugs available to American consumers throughout the life cycle of these drugs.
“Lean Pharmacovigilance streamlines and optimizes the process of receiving, reviewing, assessing and reporting adverse events from medications produced and administered during the different phases of clinical trials and post-marketed studies.”
Pat Lucansky, CSSMBB, CLS, CMC Executive Director, VIP Group |
Drug Safety Enhanced By Lean Culture
Due to significant changes in oversight and compliance measures for drug manufacturers, Pharmacovigilance is rapidly changing the pharmaceutical industry—because it is helping cultivate an increased understanding of factors affecting drug liability and consumer safety.
Lean Pharmacovigilance applies the proven and trusted Six Sigma Lean Enterprise Process to drug safety oversight and compliance. Applying Lean Pharmacovigilance to your company’s culture can help achieve:
Lean Pharmacovigilance applies the proven and trusted Six Sigma Lean Enterprise Process to drug safety oversight and compliance. Applying Lean Pharmacovigilance to your company’s culture can help achieve:
- Increased productivity up to 2- 3 times of existing staff levels
- Improved quality and on-time delivery averages +98%
- Co-location of resources reducing process times and distance traveled
- Compliance improves to an average of 98%
- Minimized risk associated with non-compliant operations
Our Lean Pharmacovigilance Strategy’s Main Areas of Focus
|
The successful implementation and normalization of a Lean Pharmacovigilance culture requires a thorough understanding of, and focus on, the medical manufacturing, regulatory, and technological aspects of your company. Our Lean Enterprise specialists can assess how efficiently your current drug safety protocols function based on various factors including:
|
Lean Pharmacovigilance Transforms Your Company’s Culture
Our lean enterprise specialists can implement a Lean Pharmacovigilance culture in your company, based on the in-depth assessment of your current drug safety protocols and processes. We will work with your project management team to employ a comprehensive, scaffolded process to help educate your employees on the collective and shared benefits of a lean culture. The results of this process include:
- Clearly defined processes
- Accountable process ownership
- Clearly defined definitions and terms
- Streamlining of activity
- Periodic assessments and checks
- Syncing and sharing of databases and relevant material
- Visibly defined measures of volume, quality and costs
- Prioritizing of cases based on risk and need
- Faster data processing
- Process-based alignment of departments and teams
- Lower employee turnover
- On-time, complete and legible receiving data
Why Outsource Your Lean Pharmacovigilance Needs?
Pharmaceutical manufacturers of all sizes and specialties can greatly benefit from trusting their Lean Pharmacovigilance needs to a company that specializes in designing and implementing proven, successful lean enterprise strategies. The need for various experts in the medical, regulatory, and technologic aspects is critical to setting up a Lean Pharmacovigilance system. Our lean enterprise specialists fulfill this need, and we can provide your company and employees with our industry expertise, systems knowledge, flexibility, and customization.
By working hands-on with your project management staff, our lean enterprise team helps implement and normalize a lean culture in your company on all levels and in every department. Because the structuring and selling of a lean culture to your employees greatly impacts its success rate, you cannot afford not to trust this process to a group of lean culture experts with a solid track record of successfully implementing Lean Pharmacovigilance systems in pharmaceutical companies just like yours.
By working hands-on with your project management staff, our lean enterprise team helps implement and normalize a lean culture in your company on all levels and in every department. Because the structuring and selling of a lean culture to your employees greatly impacts its success rate, you cannot afford not to trust this process to a group of lean culture experts with a solid track record of successfully implementing Lean Pharmacovigilance systems in pharmaceutical companies just like yours.
About VIPGroup’s Lean Pharmacovigilance Experts
Value Innovation Partners Ltd. specializes in Lean Pharmacovigilance implementation for pharmaceutical organizations of all sizes and specificities. Our efficiency experts are known throughout the pharmaceutical industry for their expertise, precision, professionalism and high success rate. We have helped implement and maintain streamlined operations, industrial, manufacturing and customer service-based practices in healthcare organizations just like yours. Contact us today to see how your pharmaceutical company, your customers, and your bottom line can immediately benefit from our experience.